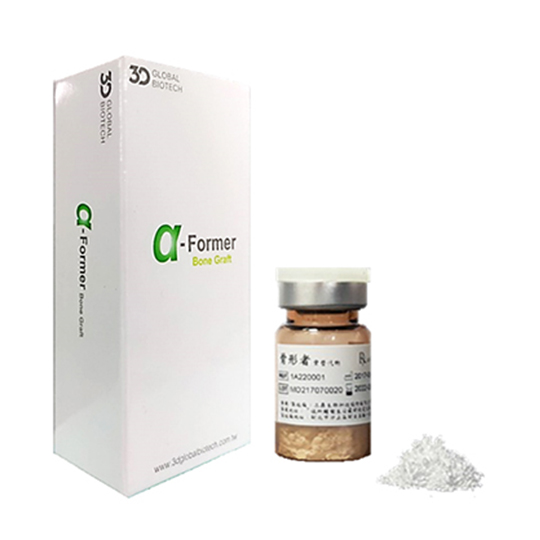
Alpha-Former®
Product Specifications
| Model(Powder) |
Specification |
| 1A220001 |
2g |
| 1A220002 |
1g |
| 1A220003 |
0.5g |
| 1A220020 |
0.25g |
| Model(Block) |
Size |
Specification |
Model(Block) |
Size |
Specification |
| 1A220008 |
0.25~0.5 mm |
2.0g |
1A220014 |
0.5~1.0 mm |
0.5g |
| 1A220009 |
0.25~0.5 mm |
1.0g |
1A220015 |
0.5~1.0 mm |
0.25g |
| 1A220010 |
0.25~0.5 mm |
0.5g |
1A220016 |
1.0~2.5 mm |
2.0g |
| 1A220011 |
0.25~0.5 mm |
0.25g |
1A220017 |
1.0~2.5 mm |
1.0g |
| 1A220012 |
0.5~1.0 mm |
2.0g |
1A220018 |
1.0~2.5 mm |
0.5g |
| 1A220013 |
0.5~1.0 mm |
1.0g |
1A220019 |
1.0~2.5 mm |
0.25 |
| Share to: |
分享
|
|
|
 |
 |
Product Description
All osteoporotic bone substitutes are packaged in sterile packaging, are intended for single patient use only, and are single-use products. Osteomorph bone substitute is composed of calcium sulfate. The packaging box contains a small bottle of calcium sulfate. If it is used as a powder, the powder should be mixed with sterile water. (Recommendation: The ratio of water to powder is 3:5) After being used according to the label instructions, the bone substitute is gradually absorbed by the body and replaced by new bone tissue within about 30 to 45 days.
|
Indications
Osteomorph bone substitutes are suitable for filling bone defects and bone defects in non-stress-bearing areas of the bone structure. Indications include:
- Printing space (length X width X height):150 mm X 150 mm X 150 mm 土 0.1
- Bone defects and bone defects caused by surgery.
- Bone defects and bone defect filling caused by trauma, plastic surgery, tumor resection or craniofacial surgery.
|
Instructions
Ministry of Health and Welfare License
Journal article publication